Lithium batteries have the advantages of portability and fast charging, so why are lead-acid batteries and other secondary batteries still circulating in the market?
In addition to the problems of cost and different application fields, another reason is security.
Lithium is the most active metal in the world. Because its chemical characteristics are too active, when lithium metal is exposed to the air, it will have a fierce oxidation reaction with oxygen, so it is prone to explosion, combustion and other phenomena. In addition, redox reaction will also occur inside the lithium battery during charging and discharging. Explosion and spontaneous combustion are mainly caused by the accumulation, diffusion and release of lithium battery after heating. In short, lithium batteries will generate a lot of heat during the charging and discharging process, which will lead to the rise of the internal temperature of the battery and the uneven temperature between individual batteries, thus causing the unstable performance of the battery.
Unsafe behaviors of thermal runaway lithium-ion battery (including battery overcharge and overdischarge, rapid charge and discharge, short circuit, mechanical abuse conditions, high temperature thermal shock, etc.) are likely to trigger dangerous side reactions inside the battery and generate heat, directly damaging the passive film on the negative electrode and positive electrode surface.
There are many reasons for triggering thermal runaway accidents of lithium ion batteries. According to the characteristics of triggering, it can be divided into mechanical abuse triggering, electrical abuse triggering and thermal abuse triggering. Mechanical abuse: refers to acupuncture, extrusion and heavy object impact caused by vehicle collision; Electric abuse: generally caused by improper voltage management or electrical component failure, including short circuit, overcharge and overdischarge; Heat abuse: caused by overheating caused by improper temperature management.
These three triggering methods are interrelated. Mechanical abuse will generally cause deformation or rupture of the battery diaphragm, resulting in direct contact between the positive and negative poles of the battery and short circuit, resulting in electrical abuse; However, under the condition of electricity abuse, the heat generation such as Joule heat increases, causing the battery temperature to rise, which develops into heat abuse, further triggering the chain type heat generation side reaction inside the battery, and finally leading to the occurrence of battery heat runaway.
Battery thermal runaway is caused by the fact that the heat generation rate of the battery is much higher than the heat dissipation rate, and the heat is accumulated in a large amount but not dissipated in time. In essence, “thermal runaway” is a positive energy feedback cycle process: the rising temperature will cause the system to become hot, and the temperature will rise after the system becomes hot, which in turn will make the system become hotter.
The process of thermal runaway: when the battery internal temperature rises, the SEI film on the surface of the SEI film decomposes under high temperature, the lithium ion embedded in the graphite will react with the electrolyte and the binder, further pushing the battery temperature up to 150 ℃, and a new violent exothermic reaction will occur at this temperature. When the battery temperature reaches above 200 ℃, the cathode material decomposes, releasing a large amount of heat and gas, and the battery starts to bulge and continuously heats up. The lithium embedded anode began to react with the electrolyte at 250-350 ℃. The charged cathode material starts to undergo violent decomposition reaction, and the electrolyte undergoes violent oxidation reaction, releasing a large amount of heat, generating high temperature and a large amount of gas, causing combustion and explosion of the battery.
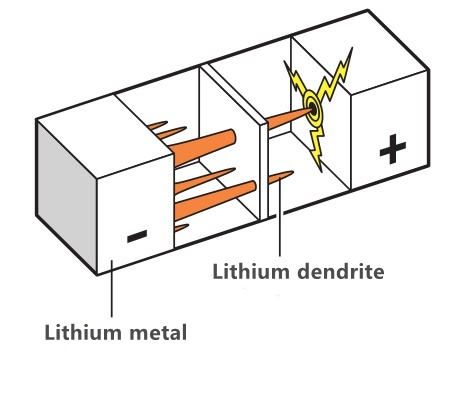
The problem of lithium dendrite precipitation during overcharge: After the lithium cobalate battery is fully charged, a large amount of lithium ions remain in the positive electrode. That is to say, the cathode can not hold more lithium ions attached to the cathode, but in the overcharged state, the excess lithium ions on the cathode will still swim to the cathode. Because they can not be fully contained, metal lithium will form on the cathode. Since this metal lithium is a dendritic crystal, it is called dendrite. If the dendrite is too long, it is easy to pierce the diaphragm, causing internal short circuit. As the main component of electrolyte is carbonate, its ignition point and boiling point are low, so it will burn or even explode at high temperature.
If it is a polymer lithium battery, the electrolyte is colloidal, which is prone to more violent combustion. In order to solve this problem, scientists try to replace safer cathode materials. The material of lithium manganate battery has certain advantages. It can ensure that the lithium ion of the positive electrode can be completely embedded into the carbon hole of the negative electrode under the full charge state, instead of having certain residues in the positive electrode like lithium cobalate, which to some extent avoids the generation of dendrites. The stable structure of lithium manganate makes its oxidation performance much lower than that of lithium cobalate. Even if there is an external short circuit (rather than an internal short circuit), it can basically avoid combustion and explosion caused by lithium metal precipitation. Lithium iron phosphate has higher thermal stability and lower oxidation capacity of electrolyte, so it has high safety.
The aging attenuation of lithium ion battery is manifested by capacity attenuation and internal resistance increase, and its internal aging attenuation mechanism includes loss of positive and negative active materials and loss of available lithium ions. When the cathode material is aged and decayed, and the capacity of the cathode is insufficient, the risk of lithium evolution from the cathode is more likely to occur. Under the condition of over discharge, the potential of cathode to lithium will rise to above 3V, which is higher than the dissolution potential of copper, causing the dissolution of copper collector. Dissolved copper ions will precipitate on the cathode surface and form copper dendrites. Copper dendrites will pass through the diaphragm, causing internal short circuit, which seriously affects the safety performance of the battery.
In addition, the overcharge resistance of aging batteries will decrease to a certain extent, mainly due to the increase of internal resistance and the decrease of positive and negative active substances, resulting in the increase of joule heat during the overcharging process of batteries. Under less overcharging, side reactions may be triggered, causing the thermal runaway of batteries. In terms of thermal stability, lithium evolution from the cathode will lead to a sharp decline in the thermal stability of the battery.
In a word, the safety performance of the aged battery will be greatly reduced, which will seriously endanger the safety of the battery. The most common solution is to equip the battery energy storage system with a battery management system (BMS). For example, the 8000 18650 batteries used in Tesla Model S can realize real-time monitoring of various physical parameters of the battery, evaluate the battery use status, and conduct online diagnosis and early warning through its battery management system. At the same time, it can also perform discharge and pre charge control, battery balance management and thermal management.
Post time: Dec-02-2022